What are the different layers in the atmosphere, and how do they differ? Layers in the atmosphere
All our weather – clouds, rain, wind, and most of the mass of the atmosphere is found in the bottom layer, the troposphere. This extends from the surface of the Earth up to about 10km – higher in the Tropics than near the poles. The air in the troposphere is warmest near the ground and is colder with height.
Above this, the stratosphere extends up to about 50km. The stratosphere is where ozone is formed (and destroyed), and temperature increases with height.
The mesosphere, thermosphere and exosphere are found above, with ever smaller concentrations of air.
In the ionosphere, an area covering the top of the thermosphere and the bottom of the exosphere, the Sun’s radiation causes the gas molecules to become positively or negatively charged. When this layer is disturbed, we see the Northern Lights are found.
Why are there different layers in the atmosphere?
It’s basically down to a question of stability – if a parcel of air rises (maybe because it’s being pushed over a mountain), cooling as it expands, does it end up cooler or warmer than the air around it? If it’s cooler, it will tend to sink back down to where it came from (it’s stable), if it’s warmer, it will tend to carry on rising (it’s unstable).
The troposphere is warmest near the ground, where radiation from the sun is absorbed. Heat is transmitted to the air by conduction and radiation as well as through evaporation and subsequent condensation of water vapour. The tropospheric air cools with height, so it is possible for parcels of air forced to rise from low down to end up warmer than the air around them, making them unstable. This gives us the clouds and rainfall that we are used to. However, the ozone in the stratosphere absorbs incoming solar radiation, and reemits it as heat, warming the upper stratosphere up. This means that, in the stratosphere, temperatures increase with height and the stratosphere is therefore always stable. All parcels of air which are pushed upwards, immediately tend to bob back down again. The stratosphere is therefore a lot less turbulent and mixed.
Does what happens in one layer have any effect on what happens elsewhere?
Yes. The most obvious example would be the effect of releasing CFCs in the troposphere on the concentrations of ozone in the stratosphere, and the subsequent consequences for the amount of ultraviolet radiation getting through to the surface of the Earth.
How does information get passed between the layers?
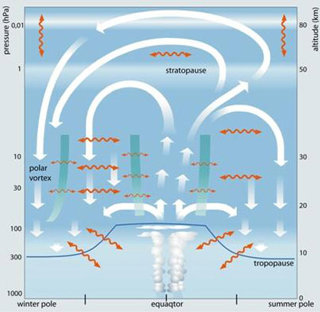
Figure: Schematic representation of the Brewer-Dobson circulation. Source
This is relatively poorly understood and the area of much active research. Actual matter (air molecules and particles), electromagnetic radiation and other forms of energy can be exchanged between the layers.
A very small amount of air moves from the troposphere into the stratosphere in the tropics. The tropics receive most energy from the Sun per unit area than the rest of the world. This heats the ground, and subsequently the air above it. Warm air rises, and we get the vigorous convection, clouds and rain we associate with the Inter Tropical Convergence Zone. The up draughts of air in some of the cumulonimbus clouds are strong enough to be able to push relatively moist air through the tropopause into the stratosphere – the air then gradually moves around in the Brewer Dobson circulation. In the same way, very large volcanic eruptions, particularly in the Tropics, can push ash and soot particles into the stratosphere.
The biggest events in the stratosphere are the so-called sudden stratospheric warmings (SSWs). They usually occur over the North Pole in winter. The polar vortex – a cyclonic vortex of stratospheric westerlies surrounding the winter pole, and enclosing the coldest air temperatures, sinking air and high surface pressure, suddenly, over the course of a couple of days, slows down or even reverses. Stratospheric temperatures over the Arctic then rise by as much as 50°C. SSWs are caused by the effects of waves propagating up from the troposphere and releasing their energy in the stratosphere (in the same way that breaking ocean waves release their energy, moving beach materials and creating noise). These waves are vast Rossby waves – with just a few wavelengths being enough to circle the globe. SSWs can have an important impact on the distribution and amount of ozone in the stratosphere.
Where in the atmosphere do you find ozone?
Zero per cent of ozone is found in the troposphere, where it is produced by the combustion of fossil fuels and has a toxic effect on animals and vegetation.
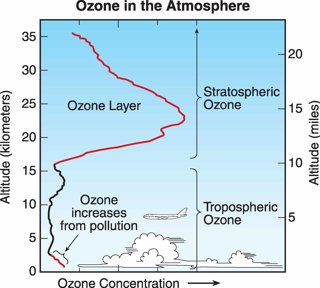
Taken from the 2006 Scientific Assessment of Ozone Depletion, WMO Image Source

Polar stratospheric clouds, taken from a British Antarctic Survey base on 27.07.07
Most ozone, about 90%, is found in the stratosphere. It is mainly formed in the tropical stratosphere and transported polewards. Ozone is extremely important to life on Earth as it absorbs ultraviolet radiation (UV-B) radiation from the Sun which can cause cell damage. In the pre-industrial world, stratospheric ozone concentrations would have increased with latitude, peaking at the Poles.
However, since industrialisation, people have been emitting ozone depleting chemicals, such as Chlorofluorocarbons (CFCs). These gases are very long-lived and so become well mixed in the troposphere and slowly are transported into the stratosphere, where the Sun’s energy converts them into more reactive gases, for example chlorine and bromine, and they destroy ozone. The chemical reactions which most effectively destroy ozone can only happen in very cold temperatures – so cold that polar stratospheric clouds form. These clouds, which are not clouds of water vapour but of nitric acid, are usually found in the within the Antarctic (and Arctic) polar vortices. Instead of having the highest concentrations, the polar regions therefore currently have virtually no ozone in the stratosphere, in winter at least.
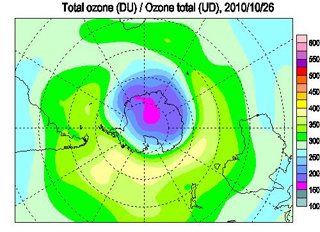
 Total ozone above the Antarctic on 26 October 2010 – clearly showing the ozone hole which is at its worst at the beginning of Spring.
Total ozone above the Antarctic on 26 October 2010 – clearly showing the ozone hole which is at its worst at the beginning of Spring.
Is the increase of greenhouse gases in the troposphere having any impact on the recovery of the ozone hole?
As the concentration of greenhouse gases in the troposphere increases and the troposphere warms, the stratosphere actually gets colder – think of putting a woolly jumper on, it keeps you warmer, but the air around you becomes cooler as you lose less heat. As the stratosphere cools, more polar stratospheric clouds form in the winter and the loss of ozone is exacerbated. In some years, all the ozone over a wide range of altitudes within the Antarctic region has gone by spring.
As the ozone hole recovers, what impact will it have on the climate we experience?
The effects on the greenhouse effect are quite complex.
Ozone is an important gas for the atmosphere, because it absorbs electro-magnetic radiation in two bands – it absorbs incoming ultraviolet radiation from the sun as well as being a greenhouse gas, absorbing outgoing infra-red (heat) radiation from the Earth. This means that, as the ozone hole recovers, less incoming radiation gets through to the surface – with benefits for all living things that do not tolerate UV radiation well. On its own, this would lead to a cooling. However, the increased ozone will contribute to the greenhouse effect – ozone absorbs wavelengths of radiation that are not intercepted by water vapour or carbon dioxide and re-emits them down to the surface of the Earth. Also, the gases currently responsible for destroying stratospheric ozone are themselves greenhouse gases; as their concentrations fall in response to international agreements, their contribution to the greenhouse effect also falls.
To add to all this, the contribution to the greenhouse effect made by ozone in the troposphere far outweighs all of the above.
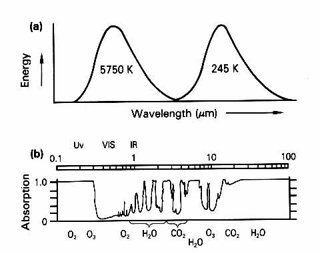
(a) Energy received and transmitted by the earth. (b) Absorption by various gases (after Houghton). Image source
What are the current best estimates for when the ozone hole will recover?
As a result of the Montreal Protocol, the effective abundance of ozone depleting gases should fall to the levels seen before the Antarctic ozone hole began to form in the 1980s by the 2050s.
Stratospheric ozone concentrations are expected to return to pre-1980 values soon after, depending on whether countries stick to emission agreements and, to some extent, on what the Sun and big volcanoes do.
What are the career opportunities for people interested in climatology?
For a career in atmospheric science (whether that’s atmospheric physics, climate science or chemistry) you really need a good science or maths degree. However, there are some opportunities for studying climate, and particularly the impacts of climate and climate change, for people with a background in geography. Similarly most people in the weather forecasting industry will have either an undergraduate or masters degree in science. There is a lot of information about the sorts of jobs meteorologists have on the Metlink website.
Sylvia was interviewed in July 2011.
Dr Sylvia Knight's Biography
Dr Sylvia Knight FRMetS joined the Royal Meteorological Society as Head of Education in 2007, since when her working life has been incredibly varied and interesting. Her career in meteorology started when, after completing a degree in Natural Sciences at Cambridge University, she started a PhD at Reading University modelling the development of waves in different kinds of atmospheric flow. After this she spent three years using a state-of-the-art computer model to investigate how changes in atmospheric greenhouse gases and ozone interact with stratospheric temperature, then moved on to work with the climateprediction.net project, distributing climate models to be run as screensavers on home computers.




